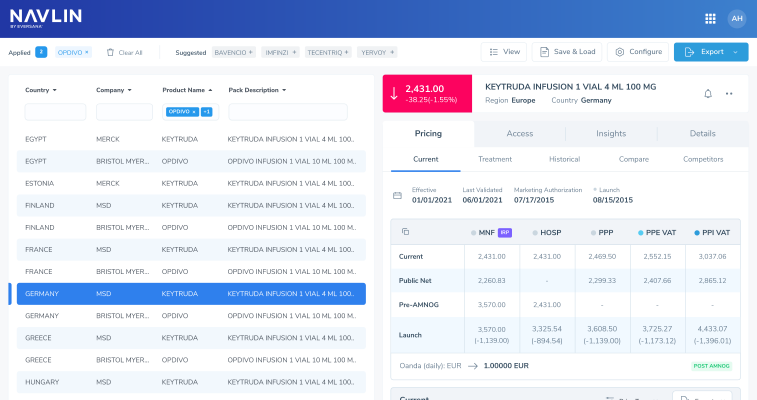
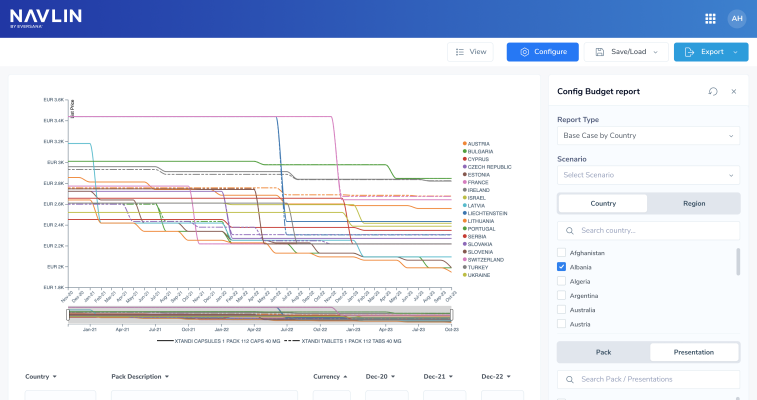
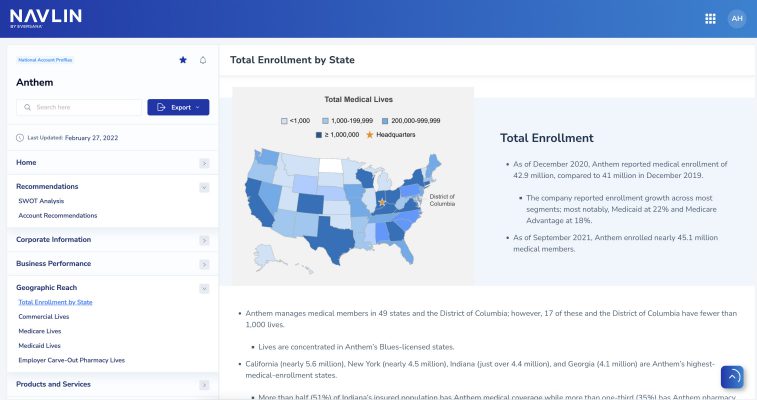
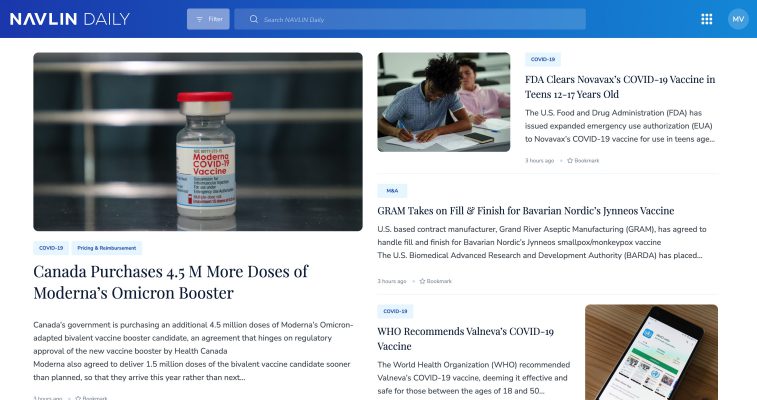
NAVLIN Daily NewsCheckout Today’s News

In response to the Inflation Reduction Act (IRA), Diana Brixner from the University of Utah, thinks companies will evolve R&D strategies: “Blockbuster drugs will be looked at differently,” she said at ISPOR 2024, adding that drug developers will make decisions on pipelines and investments according to negotiations by drug and not by indication; Likewise, in Europe, Brixner foresees many fallouts from direct price negotiation including decreased investment in drug innovationChristopher Teale from TealeHeath sees a plethora of issues with the JCA, like complex methodologies, unrealistic timelines, insufficient expertise, and disconnected price setting at the national level; According to Teale, the JCA only indirectly influences access, because access depends on national initiatives, which may be easier to implementChanges in both the U.S. and Europe create uncertainty for drug manufacturers, according to Sam Mettam from Jazz Pharmaceuticals, who recommends that players figure out how to minimize the impact of mistakes and maximize the amount of evidence generated per dollar
At ISPOR 2024, Lou Garrison, PhD, Office of Health Economics, Seattle, WA, questioned the motives of the U.S. Centers for Medicare & Medicaid Services (CMS): “Most HTA processes talk about price at launch, not 10 years later like CMS, when there are 10 years of data…Maybe there is a hidden agenda to negotiate at launch, so this is to put the camel in the tent”Regarding potential discrimination in QALY, Garrison blames concerns on politics: “QALY technically could discriminate against life expectancy, but we have mathematical ways to adjust it to favor people with certain conditions. There's a workaround”The National Health Council (NHC) recently led a roundtable for patient community members to devise a way forward for developing patient input opportunities at the CMS which is now considering combination sessions for drugs that treat similar conditions, and live-streamed meetings
At ISPOR 2024, Marco Boeri, PhD, Open Health, said, “There is a link between value, preferences, and behaviors that is not always clear to researchers and practitioners”For example, Brett Hauber, PhD, Pfizer, asked if medication taking can be an “economic bad,” as he observed patient burdens like hard-to-swallow pills, injection aversion, and prescription management—In some disease areas like cancer, people are willing to trade off actual years of life for a preferred mode of administrationWhen asked why a payer should care about patient well-being, Brett Hauber, PhD, Pfizer, answered, “I don't know if they should. All of these presentations today are saying, let's imagine suboptimal health outcomes are rational. We've left open what that means for payers”
At ISPOR 2024, Andrew York, PharmD, JD, Maryland Prescription Drug Affordability Board, warned that states have a smaller scope and less expertise than the federal government in cell and gene therapy reimbursement negotiationsBreakeven cumulative cost differences of CGTs versus standard of care have been found, but sometimes equity issues prevent breakeven points during a feasible contract periodYork believes the federal government should control the infrastructure for widespread real-world data collection
Uncertainty surrounds how the Centers for Medicare and Medicaid Services (CMS) will piece together information to inform initial offer prices and negotiations, but the CMS suggested the process would evolve as it learns and develops its capabilitiesExperts at ISPOR 2024 said CMS guidance released last week describes a predominantly qualitative approach, with one panelist calling the prioritization of focus groups encouragingImportant aspects stressed by the panel were the quantification of public input and meaningful inclusion of patient experience—not just from advocates
At ISPOR 2024, Riku Ota, MPH, Novo Nordisk, København, Denmark, warned that a lack of consideration of health equity in HTA could limit access to treatments and cause misalignments in resource allocationFor adopting equity into Cost-Effectiveness Analysis (CEA), the GRACE (Generalized Risk-Adjusted Cost-Effectiveness) approach is sometimes favoredEquity-informed economic evaluation, however, should not be used to replace or substitute the deliberate process of HTA, said multiple panelists
The quality-adjusted life year (QALY), a global gold standard for measuring health gains based on cost-effectiveness, has been denounced by academics and banned from U.S.-based decision-makingAfter experts presented the pros and cons of three alternatives to QALY (evLY, GRACE, and HYT), the audience voted in favor of GRACE by a landslideBut an audience member and former policymaker said GRACE is difficult to understand, adding that legislators are not health economists
Speaking at Reuters Pharma Europe 2024, CSL Behring’s SVP & General Manager of Commercial Operations in Europe, Lutz Bonacker, explored the company’s learnings in bringing a gene therapy to market in EuropeGene therapy commercialization is complex and nuanced, particularly in the context of big shake-ups in the European environment. CSL has Europe-based experience with Hemgenix (etranacogene dezaparvovec), a hemophilia gene therapy, for which it uses an annual, adaptive payment model in Germany to balance financial impact and evidence uncertaintyNAVLIN Daily sat down with Bonacker at Reuters to discuss the implications of legislative changes in Europe, as well as the significance of the upcoming EU HTA regulation (HTAr) for gene therapy commercialization
Join us at NAVLIN’s Pharma Pricing Innovation Conference (PPIC), which will take place on June 4-6 at the Movenpick in Basel, SwitzerlandJoin a cohort of peers and industry executives at the event’s 2024 incarnation, hosted by NAVLIN by EVERSANAConference registration includes access to pre-conference workshops, the two-day conference, exclusive networking events, and dinners. After the event, copies of all presentations will be available to attendees
Speaking at the World Orphan Drug Congress (WODC) 2024 conference, Mark Trusheim, Strategic Director, MIT NEWDIGS, discussed the importance of innovative payment models for securing payer coverage of therapies for rare diseasesTrusheim noted that while collectively, rare disease treatments make up just a small fraction of overall health care spending in the U.S., their high upfront costs can present a financial challenge to payers. Small payers and self-insured employers are especially vulnerableThe NEWDIGS initiative identified five financing solutions designed to address concerns around covering durable therapies. These include (1) short-term, milestone-based contracts; (2) multi-year performance-based annuities; (3) warranty models; (4) stop-loss/reinsurance and subscription/Netflix models; and (5) orphan reinsurer and benefit manager (ORBM) and risk pools